임상개발 CRO
Clinical Operation Service
위수탁 업무
- Study Design Consulting
- Site Management
- Protocol Development
- Site Monitoring Visit
- Regulatory Affairs (IND)
- Safety Management
- Feasibility (Site/Investigator)
- Close-Out visit
- Pre-Study Site Visit
- Data Management
- IRB Affairs
- Statical Analysis
- Site Contrast Support
- Clinical Study Report Writing
- Site Initiation Visit
- Project Management
Specialty Area
- 의약품 1상, 4상
- 의료기기
- 한의학
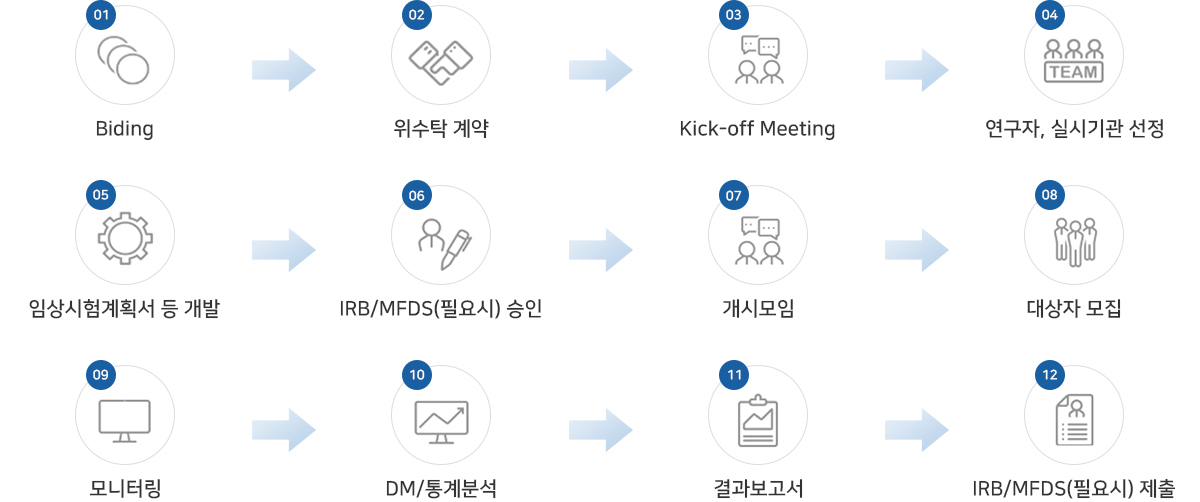
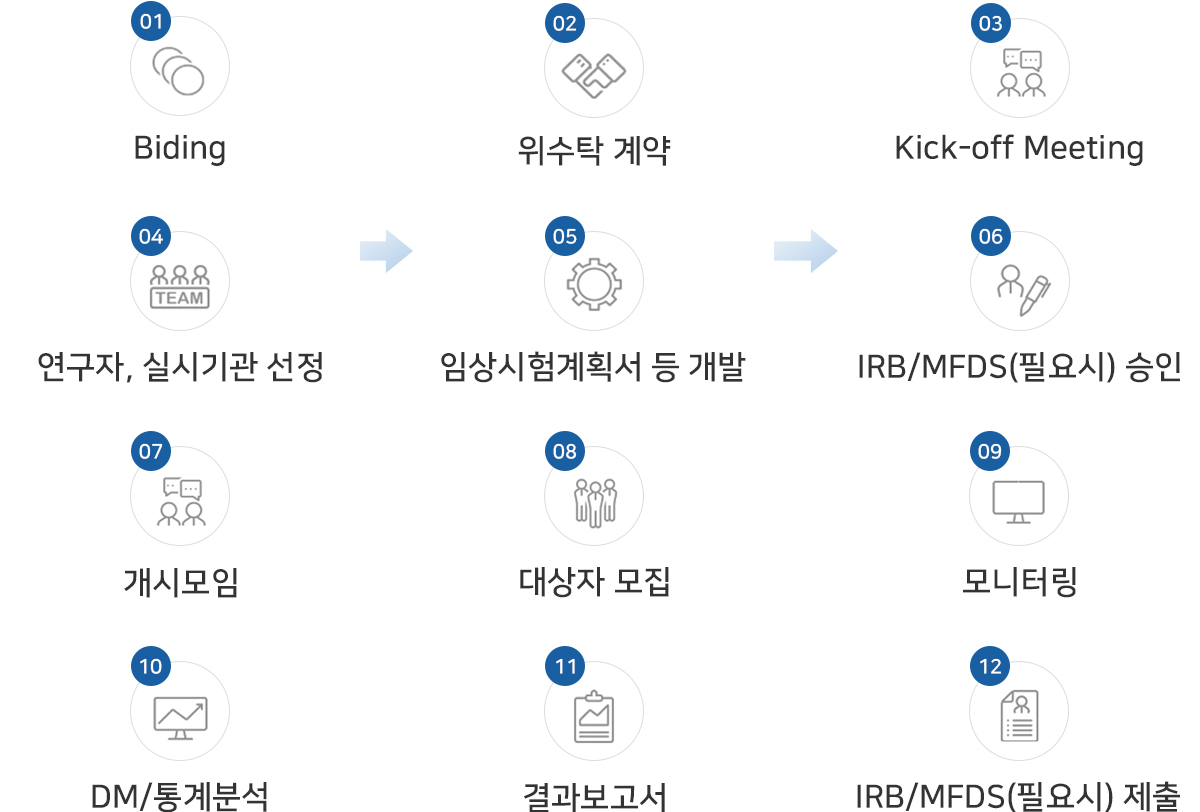
업무절차